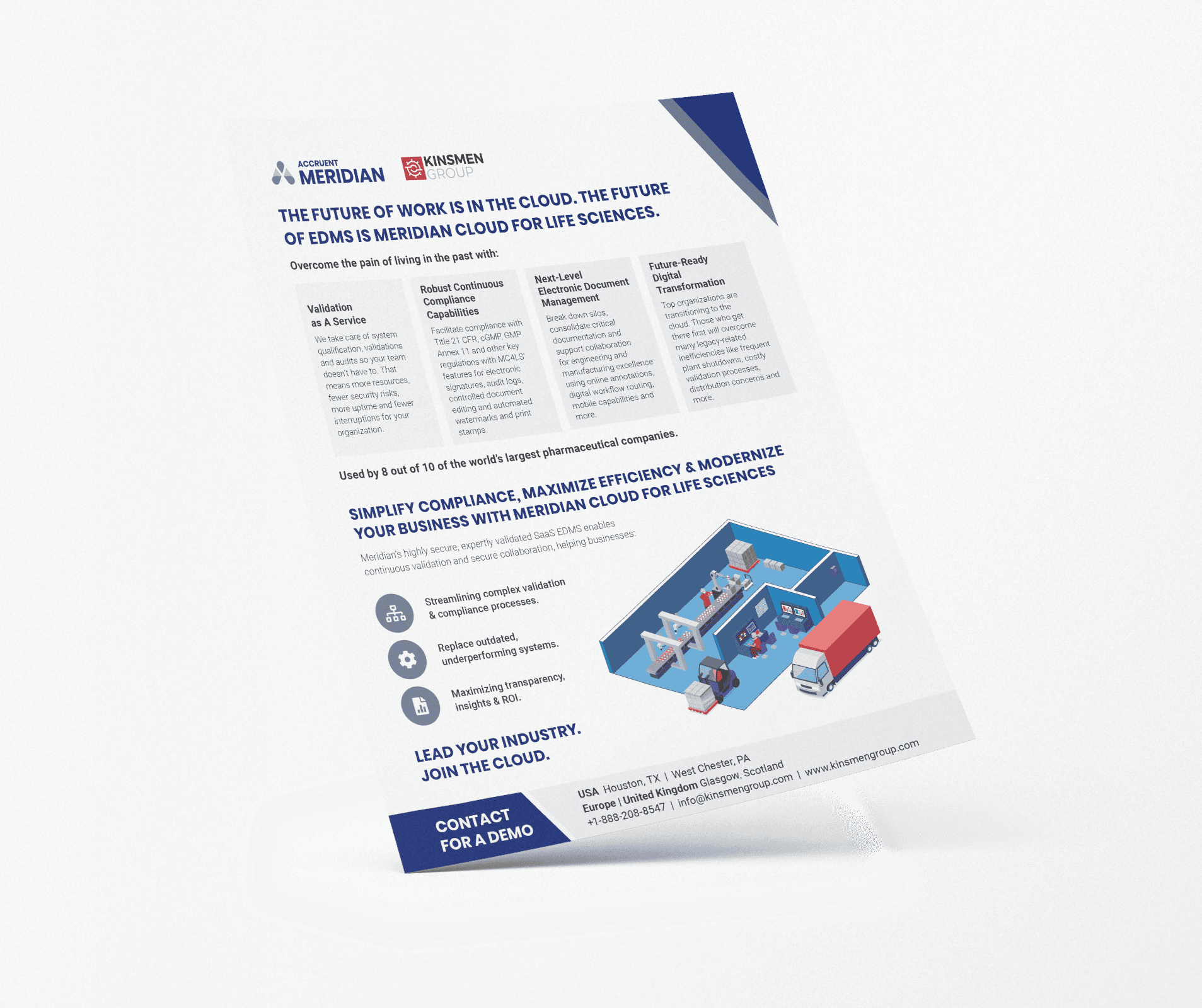
Meridian streamlines the management of technical documents and drawings throughout their lifecycle, providing a centralized repository for organizing and controlling critical data. Designed to navigate complex regulations like FDA 21 CFR Part 11, Meridian ensures compliance while empowering pharmaceutical companies to innovate and build collaborative, cutting-edge facilities.
Meridian helps your teams:
- Share the most accurate, up-to-date information
- Locate all data in a single source of truth
- Hit compliance and validation goals
- Enforce good manufacturing practices (GMP)
10 of the world’s biggest pharmaceutical companies use Meridian
Meridian transforms engineering operations – helping your teams easily manage technical documents and drawings while giving them the right information at the right time.


– Bill Eager, Drawing Management Senior Engineer, AbbVie
From real-time document visibility to configurable workflows, Meridian improves asset maintenance and document management. Centralized information makes it easy to reduce work order prep time and keep operations speedy, safe, and productive.
- Track changes and revisions to keep projects on your timetable.
- Ensure documents, project files, and drawings are accurate and accessible to the right people
- Help teams make informed decisions, from the factory floor to asset maintenance.
No more silos. Engineering documents and other information are centralized in a single source of truth to keep engineering, maintenance, IT, and contractors working from the same page.
- Markup tools with seamless commenting and editing empower collaboration across departments and teams.
- Access to correct, up-to-date information reduces costly errors and helps expedite production.
- Seamless integrations with EAM, ERP, ECM, Office, and CAD applications make it easy to implement across the organization quickly.
Vetted and validated by the FDA for meeting CFR compliance regulations, Meridian puts control back in your hands.
- Audit trails, system validations, and record retention gives your team peace of mind at audit time.
- Transparent, traceable data collection and reporting ensure accuracy in audit preparation.
- Security controls protect sensitive data from breaches, while satisfying compliance regulations in the United States, EU, and beyond.
Customer Use Case

When AbbVie split from Abbott, they faced the challenge of managing 130 engineering IT applications across global sites. Needing a centralized, standardized solution, AbbVie adopted Meridian. Today, over 625 employees use Meridian to manage 300,000+ CAD drawings, ensuring compliance with FDA 21 CFR Part 11, improving maintenance efficiency, and fostering collaboration across departments.
Learn More

Expertise meets excellence:
Meridian + Kinsmen Group
Kinsmen Group and Meridian have a long history of collaboration in engineering document management technology. Closely involved with the development of the FDA module in Meridian, Kinsmen Group is a leading expert in pharmaceutical technology and best practices that can help you achieve easy and flawless compliance.
Our partnership expedites both implementation and processes to help you reach new heights of information access, collaboration, and compliance excellence.
Kinsmen Group provides comprehensive Document Control and CAD Drafting services tailored to the unique needs of the pharmaceutical industry. Our Document Control services ensure the proper management, organization, and compliance of critical documents, including version control and approvals. In addition, we offer full CAD Drafting services, creating and maintaining detailed drawings for essential systems such as HVAC, electrical, plumbing, and fire suppression. Our team manages the entire Master Drawing Program, from project kickoff through regular coordination meetings and site verifications, ensuring precise and up-to-date documentation. With a focus on accuracy and efficiency, Kinsmen Group supports the successful execution of facility documentation throughout the project timeline.

at Kinsmen Group.
Learn how our engineering document management tools can help solve your data and compliance challenges.
© Kinsmen Group, LLC. | All Rights Reserved | Terms and Conditions | Privacy Policy